Scientists:
Volodymyr Shvadchak, Maksym Galkin
Challenge
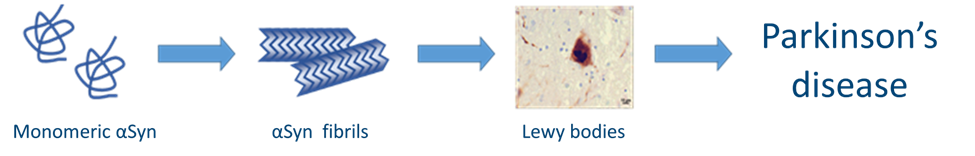
Parkinson's disease (PD) is a neurodegenerative disorder that impacts over 1% of individuals aged 60, and more than 4% of those aged 80 and above. Despite the increasing demand, a cure for PD remains elusive, with only symptomatic treatments currently available. The progression of PD is heavily driven by the aggregation of small protein α-synuclein (αSyn) into amyloid fibrils that makes stopping this process a prospective strategy for the disease treatment.
Technology
Amyloid fibrils grow by sequential binding of αSyn to their ends. Developed peptide-based inhibitors selectively bind to the fibril ends and block attachment of αSyn preventing amyloid formation.
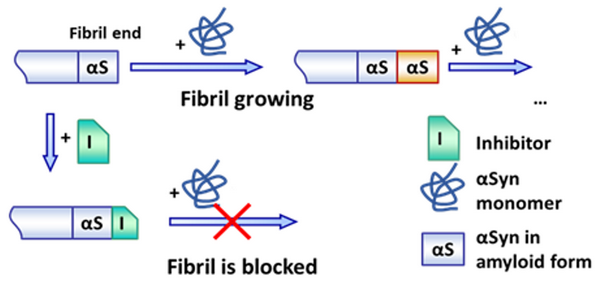
Therefore, the inhibitors were named FEPI – Fibril End Peptide Inhibitors. They were tailored to recognize the structural motif of fibril ends. This design allows for selectivity for pathological species not affecting protein in its native form. As a result, the lead peptide FEPI4 prevents aggregate formation in cells with much higher efficacy than any other compounds reported to date.
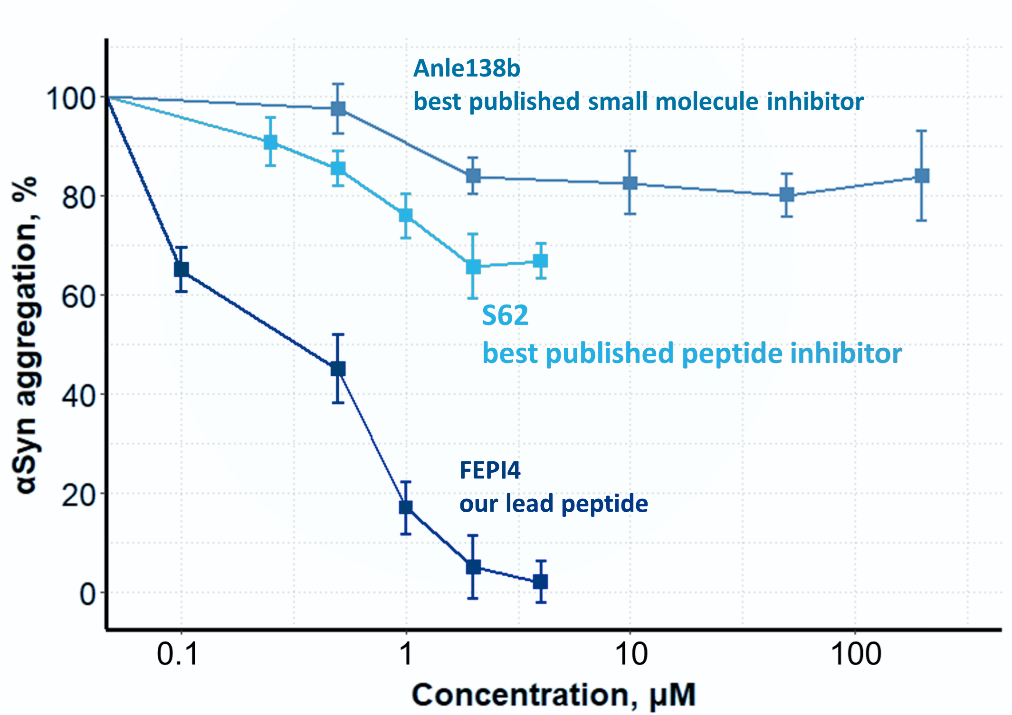
αSyn aggregation in cells after 24h treatment with the inhibitor as compared to control cells
FEPI were modified for effective intranasal delivery to the brain - a less common but effective route, which transfers biomolecules through the olfactory nerve to reach the brain areas affected in PD – the olfactory bulb and basal ganglia. The effective delivery of the peptides to the brain through the intranasal route was shown using a mice model.
The ultimate goal is to develop an intranasal spray suitable for self-administration that can stop PD progression.
Commercial opportunity
With the aging of the world's population, the global market for antiparkinsonian drugs is projected to triple in the next decade. In 2029 PD diagnosed population is predicted to be 3M and total sales $11.5B.
Development status
Preclinical, in vivo testing
Categories
Antiparkinsonian drugs; α-Synuclein aggregation inhibitor
Keywords
Parkinson's disease α-Synuclein aggregation amyloid fibrils FEPI